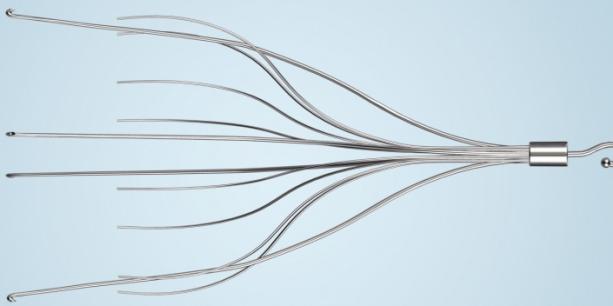
Bard inferior vena cava (IVF) filters are small medical devices with spider-like legs which are inserted into a vein in order to “catch” a blood clot before it travels to the lungs or heart. In theory, once the blood clots are trapped in the IVC filter, the clots remain in place until they dissolve. While anticoagulants are the first line of defense to break up blood clots, IVC filters are an alternative or adjunct treatment for those at risk of a pulmonary embolism. IVC filters were introduced into to the market in the 1960’s, and meant to prevent thrombosis injuries.
Bard IVC filters can be temporary or permanent; both types are implanted in the inferior vena cava, a vein in the human body responsible for returning blood to the heart from the lower extremities. Traveling blood clots typically form in the inferior vena cava, potentially causing fatal complications. There are more and more concerns being raised about Bard and other IVC filters, particularly the retrievable type. Because these IVC filters were only designed for short-term treatments, they could be responsible for life-threatening complications.
ADVERSE EVENT REPORTS ASSOCIATED WITH IVC FILTERS
The first retrievable IVC filters garnered FDA approval in 2003 and 2004. Yet from 2005-2010, the FDA received 921 adverse event reports related to the IVC filters. Of those 921 adverse event reports, 328 were for migration associated with the filter, 146 involved embolizations (detachment of device components), 70 involved perforations of the IVC, and 56 involved filter fracture. These events may relate to a retrievable filter remaining in the body far beyond the time when embolism risks have passed.
The FDA issued a warning regarding IVC filters in 2010, noting they were concerned that the filters which were intended for short-term placement were, in many cases, not being taken out in a timely manner. In this Safety Communication, as well as another sent out in 2014, the FDA cautioned those patients with retrievable filters that their complications could be minimized or altogether avoided if the filter was removed once the risk of pulmonary embolism subsided.
THE PRESERVE STUDY
In the 2014 Safety Communication update, the FDA notes publicly available medical data was used in the assessment of whether a specific time period exists in which the risks associated with an inferior vena cava filter outweighs the expected benefits. The Journal of Vascular Surgery: Venous and Lymphatic Disorders, published an article on IVC filters in the fall of 2013. The model in the article suggested a risk/benefit profile of removal of the filter from 29-54 days following implantation so long as the pulmonary embolism risk had passed.
The FDA continues to collect information and data for IVC filters currently marketed in the U.S., including those by Bard. Some manufacturers of IVC filters are participating in a study called PRESERVE. This study examines the use of inferior vena cava filters to prevent pulmonary embolism. The resulting data should help in the determination of when retrievable IVC filters should be removed and how safe the filters are.
BARD RECEIVES FDA WARNING
In addition to complications associated with its IVC Filter, C.R. Bard received notification from the FDA on July 23, 2015, that the company used their Recovery Cone Removal System without required clearance or approval, and failed to inform the FDA of serious device malfunctions. The Recovery Cone Removal System is a device used to retrieve Bard’s IVC filters. The letter to Bard also raised concerns regarding the company’s adherence to Quality System Regulations at its Tempe facility, noting a complaint related to the G2 IVC filter should have been filed as a death rather than a malfunction Medical Device Report. The types of retrievable IVC filters manufactured by Bard includes the Meridian, the Eclipse, the Bard G2 Express, the Bard G2 and the Bard Recovery IVC Filter which was withdrawn in 2005.
If you or a loved one suffered injuries after having a Bard IVC retrievable filter implanted, you may be able to seek compensation. If you would like to speak with one of our attorneys about your rights and legal options, contact our office right away by calling us at (877)-696-3303 or by filling out this form. It is in your best interests to act quickly. There is a time limit in each State for filing injury claims known as the Statutes of Limitations. If a claim is not filed against the manufacturer, marketers, retailers and healthcare providers before the statute of limitations expires, an injured party will not be able to bring a claim against those liable for their injuries.